Abstract
Introduction BMT CTN 1101 was a multicenter phase III randomized trial comparing the clinical outcomes and quality of life (QOL) of patients with hematological malignancies receiving double umbilical cord blood transplants (dUCBT) or haploidentical bone marrow transplants (haplo) with post-transplant cyclophosphamide after reduced intensity conditioning. Extended follow-up at 5 years showed that there were no significant differences in progression free survival (PFS) or overall survival (OS) between the two cohorts. However, the hazard ratio for NRM was 2.69 in favor of haplo transplants with all of the difference seen in the first 18 months post-transplant. The impact of donor source on QOL, however, is unknown. Thus, the objectives of this analysis are to compare longitudinal QOL between dUCBT and haplo and to investigate the association of QOL and clinical outcomes.
Methods Enrollment occurred from 6/2012-6/2018, and included 368 patients (n=186 dUCBT, n=182 haplo), age 18-70 with 2 year follow up. QOL assessments included the FACT-BMT measure; the MOS Short Form 36 Physical Component Score (PCS) and Mental Component Score (MCS); the EQ-5D; and the Global QOL Scale. These were completed pre-transplant and at 12 and 24 months post-transplant. To account for missing assessments, scores were compared between arms using an inverse probability weighted-independent estimating equations (IPW-IEE) method that models scores in surviving patients while accounting for baseline variables (age, race/ethnicity, primary disease, disease risk, performance status, and HCT-CI) and follow-up outcomes that impact the likelihood of missingness. Cox models evaluated potential associations of pre-transplant QOL scores with OS and PFS while adjusting for these baseline variables. Analyses were performed using intention-to-treat (ITT) patient assignment from the time of randomization. A threshold of 1% was used to determine statistical significance for all analyses.
Results No significant differences were observed between treatment arms in baseline patient, disease, and transplant characteristics (previously described) among patients who participated in at least one QOL assessment. In the UCB arm, PRO response rates were 145/186 (78%) pre-transplant, 67/105 (64%) at 12 months, and 47/85 (55%) at 24 months. In the haplo arm, PRO response rates were 134/182 (74%) pre-transplant, 79/115 (69%) at 12 months, and 50/102 (49%) at 24 months. HCT-CI >3 pre-transplant and relapse at 12 and 24 months were the most consistent factors significantly associated with lower completion of QOL questionnaires at respective time points.
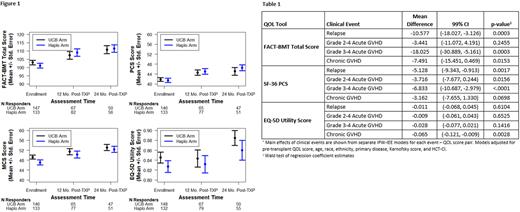
We found no significant difference in any of the QOL scores at pre-transplant, 12, and 24 months between the treatment arms (Figure 1). In multivariable IPW-IEE models, pre-transplant FACT-BMT total score, SF-36 PCS, and EQ-5D scores were the only significant predictors of post-transplant QOL scores. There was no significant association of treatment arm or baseline clinical variables with FACT-BMT or SF-36 PCS scores post-transplant. Age >60 was associated with higher mean EQ-5D (0.08, 99% CI 0.025-0.131, p=0.0002) and SF-36 MCS scores (4.45, 99% CI 0.288-8.622, p=0.0059) on post-transplant assessments. There was no significant impact of treatment arm on Global QOL score or subscale scores post-transplant.
We evaluated the association of post-transplant clinical events with primary QOL endpoints (Table 1). Relapse and Grade III-IV aGVHD were associated with significant declines in mean FACT-BMT and SF-36 PCS scores. cGVHD was associated with a decline in mean EQ-5D utility scores. We found no significant association between pre-transplant QOL scores and OS or PFS (p>0.10 for all models). There were no significant interactions between treatment groups and baseline characteristics on QOL measures.
Conclusions This longitudinal QOL analysis of BMT CTN 1101 showed that donor type did not impact post-transplant QOL. Pre-transplant QOL scores were the only predictor of post-transplant 12 and 24 month scores which, however, were not associated with OS or PFS in either treatment arm. Clinical events of GVHD and relapse were associated with declines in QOL scores.
Disclosures
Lee:Syndax: Research Funding; Novartis: Other: Steering Committee; Amgen: Research Funding; AstraZeneca: Research Funding; Incyte: Research Funding; Kadmon: Consultancy, Honoraria, Research Funding; Pfizer: Research Funding; Pfizer: Research Funding; Syndax: Research Funding; Incyte: Research Funding; AstraZeneca: Research Funding; Amgen: Research Funding; Kadmon: Consultancy, Honoraria, Research Funding; Equillium: Consultancy, Honoraria; Mallinckrodt: Consultancy, Honoraria; National Marrow Donor Program: Membership on an entity's Board of Directors or advisory committees. D'Souza:Takeda, Sanofi, TeneoBio, Prothena, Caelum Biosciences, Janssen Oncology, Regeneron, Abbvie: Research Funding; Pfizer, Janssen Oncology, Bristol-Myers Squibb/Celgene, Prothena: Consultancy, Membership on an entity's Board of Directors or advisory committees. Shapiro:Miltenyi: Honoraria. Hamilton:Syndax: Membership on an entity's Board of Directors or advisory committees; Equilium: Membership on an entity's Board of Directors or advisory committees; Nkarta: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Kadmon: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal